- Aluminum is found in varying amounts in nature as aluminosilicates (contains aluminum, silicon, and oxygen) in various types of clay. As the minerals are weathered they gradually breakdown into various forms of hydrated aluminum oxide, Al2O3.xH2O, known as bauxite.
- The bauxite is purified by the Bayer Process. First the ore is mixed with a hot concentrated solution of sodium hydroxide. The NaOH will dissolve the oxides of aluminum and silicon but not other impurities such as iron oxides, which remains insoluble. The insoluble materials are removed by filtration.
- The solution which now contains the oxides of aluminum and silicon are next treated by bubbling carbon dioxide gas through the solution. Carbon dioxide forms a weak acid solution of carbonic acid which neutralizes the sodium hydroxide from the first treatment. This neutralization selectively precipitates the aluminum oxide, but leaves the silicates in solution. Again filtration is used for the separation. After this stage the purified aluminum oxide is heated to evaporate the water.
- Aluminum in the metal form is very difficult to obtain by using some of the traditional chemical methods involving carbon or carbon monoxide as reducing agents to reduce the aluminum ions to aluminum metal. One of the earliest and costly methods in 1850 was to reduce aluminum chloride with sodium metal to obtain aluminum metal and sodium chloride. (Sodium metal is not easy to obtain either). As a result some of the earliest aluminum metal was made into jewelry.
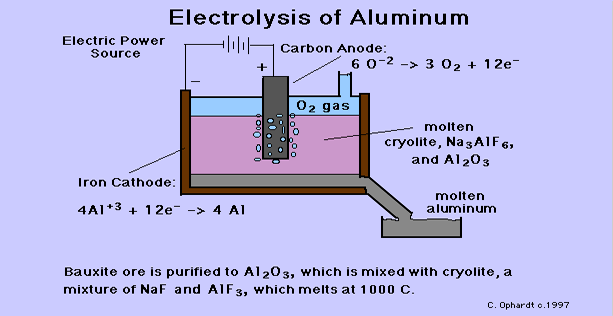
- In 1886, Charles Hall, an American (23 years. old), and Paul Heroult, a Frenchmen (23 years old), simultaneously and independently developed the process still in use today to make aluminum metal. The purified aluminum oxide is mixed with cryolite, a mixture of sodium fluoride and aluminum fluoride, and heated to about 980 degrees Celsius to melt the solids. The mixture melts at a much lower temperature than aluminum oxide would by itself.
- The hot molten mixture is electrolyzed at a low voltage of 4-5 volts, but a high current of 50,000-150,000 amps. Aluminum ions are reduced to aluminum metal at the cathode (the sides and bottom of the electrolysis cell). At the anode, oxygen is produced from the oxide ions. The anode material is carbon in the form of graphite, which also is oxidized and must be replaced quite frequently.
- The electricity used to produce aluminum is relatively high. One pound of aluminum requires 6-8 kilowatt-hours of electrical energy. This amount of aluminum can be used to make 23 pop cans or one 300 watt light bulb burning for one hour is required to make one pop can.
- Source: Aluminum Smelting-Virtual chembook Elmhurst College Charles E. Ophardt, c. 2003
-
About us
Contact us
Make a suggestion
- Metalpedia is a non-profit website, aiming to broaden metal knowledge and provide extensive reference database to users. It provides users reliable information and knowledge to the greatest extent. If there is any copyright violation, please notify us through our contact details to delete such infringement content promptly.

