Zinc
Zinc- Zn is the symbol for zinc which is a chemical element in the IIB group of the periodic table with an atomic number of 30 and atomic weight of 65.39. Its density is 7.14 g•cm−3. The melting and boiling points of zinc are 692.68K (419.53 °C, 787.15 °F) and 1180 K (907 °C, 1665 °F) respectively. Pure zinc is a bluish-white, transition-metal. It is hard and brittle at most temperatures but becomes malleable between 100-150°C. When the temperature rises above 210°C, it becomes brittle again. The surface of pure zinc tarnishes quickly, and a layer of the basic zinc carbonate is formed to prevent further oxidation. Zinc itself is a common non-ferrous metal, which can combine many other non-ferrous metals to form alloys, the main alloys being brass and die-casting alloys . Zinc and its alloys are mainly used for iron and steel, metallurgy, machinery, the electrical and chemical industries, light industry, military applications, medicine and some other fields.
Zinc is one of the essential elements for human health and it is part of the composition of more than 200 kinds of enzyme.

- Zinc: discovery and industry development
- Zinc is one of the elements best known for its compounds. It was first found and used in China. Both its English name Zinc and chemical symbol Zn come from the Latin Zincum, which means "white thin layer" or "white sediments"...

- Zinc: uses
- With good calenderability, abrasive resistance, corrosion resistance, castability, and room temperature mechanical properties, zinc can be made into various alloys with many other metals. Mainly in the form of galvanization, zinc-based alloys and zinc oxide, it has applications in the automobile, construction and shipbuilding industries...
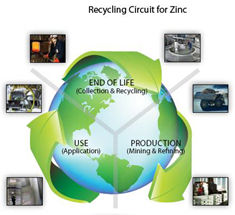
- Zinc: recycling
- In recent years, with zinc smelting technologies continually improving, consumption of zinc as a raw material has been huge. As a result, zinc ore resources have declined and metal zinc is facing serious raw material shortages. Increasingly, ores from different areas will cease to be mined in future. Therefore, responsible usage and recycling of existing resources has gradually become the focus...

- Zinc: resource distribution and production
- Metallic zinc resources are distributed widely in nature, and zinc commonly exists in sulfide state. The mineral spreads over blende (mainly), as well as a few oxidized ores like smithsonite, willemite, hemimorphite and hydrocincite. Currently, the identified zinc resources of the world are about 1.9 billion metric tonnes...

- Zinc: smelting
- There are two methods of smelting zinc: the pyrometallurgical process and the hydrometallurgical process (over 90% of hydrometallurgical process is in electrolytic plants using electrolysis process). Both methods are still used...

- Zinc: news
- It is known that zinc has long been added to denture cream to improve its adhesive properties. The FDA has received a letter which was sent to denture-adhesive manufacturers. It pointed to "numerous reports of adverse events related to the use of denture creams", which might mean zinc toxicity...
- Contents
- Reference
- Zinc--U.S. Geological Survey, Mineral Commodity Summaries, February 2014
- Zinc smelting--Wikipedia
- 12.7 Zinc Smelting--EPA
- Zinc Production - From Ore to Metal--International Zinc Association
- Zinc Recycling--International Zinc Association
- International Zinc Association (IZA)
- International Lead and Zinc Study Group
- Super Galvanising launches new continuous galvanizing rebar line (Brussels - Dubai, 20 May 2014)--International Zinc Association