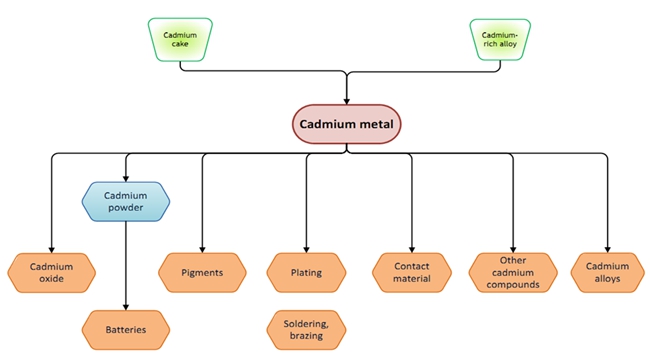
- Cadmium has many chemical and physical properties that make it desirable for industrial and consumer applications: resistance to corrosion and chemicals, tolerance of high temperatures, a low melting point and excellent electrical conductivity. These properties make cadmium suitable for use in alloys, pigments, coatings, stabilizers and rechargeable nickel-cadmium (Ni-Cd) batteries, which are the primary application. In the near future, solar cell manufacturing may become another significant market for cadmium.
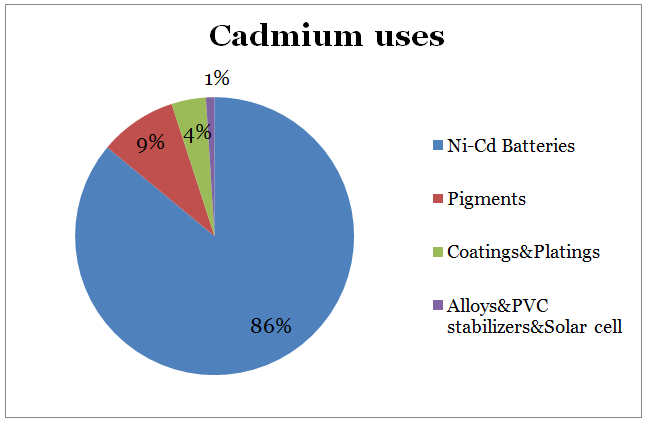
- Approximately 86% of the cadmium consumed globally was used in NiCd batteries, 9% in pigments, 4% in coatings and 1% in various uses including alloys, solar cells and stabilizers (Morrow, 2011, p. 10).
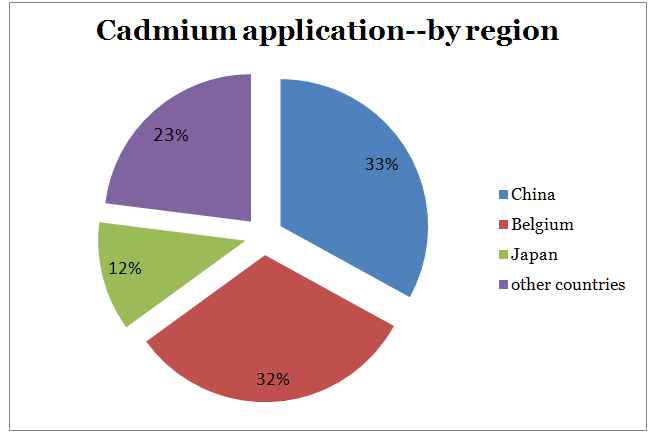
- Global refined cadmium consumption was 16,200 tons in 2011 (latest data available), a slight drop from that of 2010. China was the leading consumer accounting for 33% of consumption, followed by Belgium (32%) and Japan (11%). Most NiCd batteries are manufactured in China and, to a lesser degree, Japan. Japanese production of portable NiCd batteries dwindled by 5% in 2012 compared with 2011 to 176 million units. Total portable rechargeable battery production in Japan declined by 9% in 2012. China imported a high percentage of the global cadmium produced outside its borders; 2012 imports of unwrought cadmium totaled 12,600t. Leading importers included the Republic of Korea (35%), Kazakhstan (9%), Mexico (9%), Russia (7%), Japan (7%), and Canada (6%). In Belgium, Floridienne Chimie S.A., an intermediate processor of cadmium, made up almost all of the country’s cadmium consumption for the production of cadmium compounds (carbonate, nitrate and oxide) and powder, which were then exported to downstream consumers in Asia. It was estimated that nearly 7,200 t/yr of refined cadmium were consumed by Floridienne. In 2012, Belgium imported 3,690t of unwrought cadmium (Metal Bulletin, 2009; World Bureau of Metal Statistics, 2012, p. 21; Metal-Pages, 2013; Roskill’s Letter from Japan, 2013b).
- Nickel-cadmium batteries are characterized by their resistance to electrical abuse, high cycle lives, reliability and versatility and have found a wide range of applications. The various types of cell construction are manufactured in a wide array of sizes, capacities and shapes, and the choice of a particular battery will depend upon the application and its current load requirements.
- These applications are primarily of two types: industrial and portable batteries.
- (a) Industrial nickel-cadmium batteries
- Nickel-cadmium batteries for industrial uses are of the vented (or open) or semi-sealed type and may be of pocket plate, sintered plate or fibre structured construction. Uses for industrial batteries include railway applications such as locomotive starting, emergency braking, coach lighting and air conditioning, as well as trackside power for signaling and warning lights. Other uses include standby power for alarm systems, emergency lighting, military communications, solar energy storage, navigation equipment, military equipment and hospital operating theatres. Semi-sealed industrial batteries are important in aeronautical applications, where they are used to start engines and also to provide stand-by power for aircraft systems when the principal power source fails. After long periods of operation most vented or semi-sealed cells may require electrolyte maintenance by topping up with distilled water.

- (b) Portable nickel-cadmium batteries
- Nickel-cadmium batteries for portable use are of the sealed type and are generally of sintered plate construction. They may be of cylindrical, button or prismatic design. Sealed nickel-cadmium batteries are in great demand for use in consumer electronic equipment such as cellular telephones, portable tools, toys, camcorders and other domestic cordless appliances. They are also used for memory back-up in computing equipment, military and civil communications, emergency lighting and many other similar applications. Sealed cells require no maintenance and may be recharged up to 2000 times.

- The NiCd battery industry is almost exclusively concentrated in Asia, and leading manufacturers include BYD Co., Ltd. (China), Panasonic Corp. (Japan) and Sanyo Electric Co., Ltd. (Japan). Small portable NiCds account for 80% of the cadmium consumed for the production of NiCd batteries and are used to power consumer electronics (commonly, power tools). Large industrial NiCd batteries account for the remaining 20% of consumption and are used predominantly for aeronautical and railway applications. (Morrow, 2011, p. 10–11)

- Cadmium pigments are stable inorganic colouring agents which can be produced in a range of brilliant shades of yellow, orange, red and maroon. They are predominantly used to color engineering plastics that are processed at high temperatures, because the pigments are able to withstand elevated temperatures without degrading. But they also have significant applications in ceramics, glasses and specialist paints. The replacement of zinc or mercury for cadmium and the replacement of selenium for sulfur forms the spectrum of cadmium pigments that range from lemon-yellow to maroon.
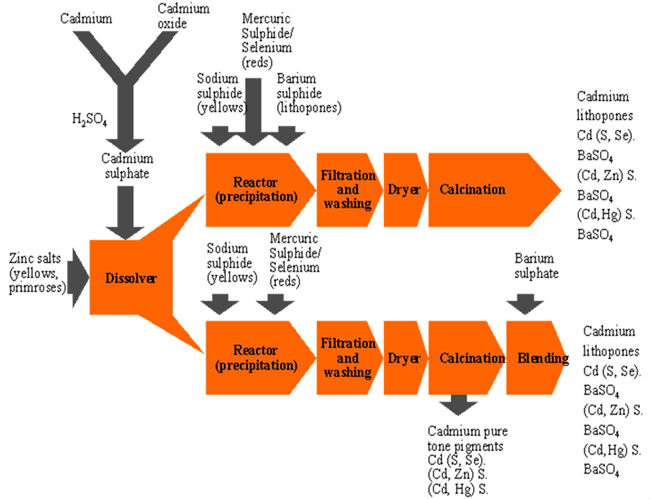
- The pigments are fine, discrete particles of coloured powder, with diameters of around 1 gm, which are distributed and suspended in the material to produce a uniformly coloured product. Cadmium pigments have excellent heat stability which makes them essential in applications where elevated processing or service temperatures are encountered.

- (a) Plastics
 Most cadmium pigments are used in plastics. These pigments disperse well in most polymers to give good colouring and high opacity and tinting strength. The pigments are insoluble in organic solvents, have good resistance to alkalis and in most cases will remain colour fast for the life of the plastic. As a result, cadmium pigments have been used in a wide range of plastic products. Currently, their primary application is in complex polymers which are processed at higher temperatures and require the unique durability and technical performance of a cadmium pigment. Their use is almost mandatory in many nylons, acrylonitrile butadiene styrenes (ABS), polycarbonates, high density polyethylenes, silicone resins and other modern thermoplastic polymers processed at high temperatures which preclude the use of organic pigments and also most alternative inorganic pigments in the range of hues provided by cadmium. Cadmium pigmented engineering polymers such as ABS are widely used in products which include telephones, gas pipes and fittings, electricity cables, beverage crates and motor vehicle radiator fans. Pigments are usually incorporated in plastics in proportions of 0.01 to 0.75 per cent by weight.
Most cadmium pigments are used in plastics. These pigments disperse well in most polymers to give good colouring and high opacity and tinting strength. The pigments are insoluble in organic solvents, have good resistance to alkalis and in most cases will remain colour fast for the life of the plastic. As a result, cadmium pigments have been used in a wide range of plastic products. Currently, their primary application is in complex polymers which are processed at higher temperatures and require the unique durability and technical performance of a cadmium pigment. Their use is almost mandatory in many nylons, acrylonitrile butadiene styrenes (ABS), polycarbonates, high density polyethylenes, silicone resins and other modern thermoplastic polymers processed at high temperatures which preclude the use of organic pigments and also most alternative inorganic pigments in the range of hues provided by cadmium. Cadmium pigmented engineering polymers such as ABS are widely used in products which include telephones, gas pipes and fittings, electricity cables, beverage crates and motor vehicle radiator fans. Pigments are usually incorporated in plastics in proportions of 0.01 to 0.75 per cent by weight.- (b) Specialist and industrial paints
- Bright cadmium yellows, oranges and reds are major pigments for artists' colours where their permanence and opacity are the accepted standards against which other pigments are judged.
- Cadmium yellows and reds can have service temperatures well above 3,000 C and are used in coatings for process chemicals and steam pipes. They can also be incorporated in latex and acrylic coatings.
- Cadmium pigments are usually incorporated in these paints in proportions of 10 to 15 per cent by weight.
- (c) Ceramics and glasses
- The unique abilities of highly stable cadmium pigments to withstand high processing and service temperatures make them the only choice in much of their colour range for glasses, ceramic glazes and vitreous and porcelain enamels. In transparent glasses the cadmium pigment particles are colloidally dispersed to produce the colours by selective absorption and scattering. The addition of 0.5 per cent by weight of cadmium pigment produces bright transparent glasses with colour ranging from intense yellow through to ruby red depending upon the cadmium sulphide to selenium ratio. A ruby red produced using a cadmium sulphide to selenium ratio of 2:1 has excellent light transmission in the red part of the spectrum with a sharp cut-off of other colours. Selenium rubies of this type are used to colour glasses for standard signal lamps for railway, marine and other uses. Greater proportions of pigment (up to about 10 per cent) are employed to make darker decorative glasses.
- The bright colours of cadmium pigments are ideally suited to ceramics, vitreous enamels for glass and porcelain enamels for iron and steel domestic products.
- (d) Other uses
- Cadmium pigments have a number of other minor uses in rubber, paper and inks, although these are small in terms of cadmium consumption.
- Cadmium coatings are applied to iron, steel, brass and aluminium and provide wonderful resistance to corrosion in most conditions and especially in marine and alkaline environments. Cadmium, like zinc, also offers sacrificial protection to a substrate such as steel by being preferentially corroded when the coating is damaged and small areas of the substrate are exposed. Apart from corrosion protection, cadmium coatings provide a low coefficient of friction and therefore good lubricity, predictable torque characteristics, good electrical conductivity, protection from galvanic corrosion (in particular when in contact with aluminium), easy solderability, low volume corrosion products and reduced risks of operating mechanisms being jammed by corrosion debris for many components in a wide range of engineering applications throughout industry.
- Cadmium coatings are particularly of use in the aerospace and military industries where they are applied to plate bolts and other fasteners in aircraft landing gear, chassis, connectors and other components. Owing to a combination of properties not present in other anticorrosive coatings, cadmium coatings are also commonly applied to parachutes. Applications in aerospace and the military are so critical that coating substitution might compromise operational safety.

- Electroplating makes up over 90 per cent of all cadmium consumed in coatings but mechanical plating and vacuum or ion deposition are also of some commercial significance. The coating is normally defined in thicknesses between 5 and 25 mm, the choice hinging on the severity of the atmosphere. Chromate post-treatment of the coating can increase coating life.
-
About us
Contact us
Make a suggestion
- Metalpedia is a non-profit website, aiming to broaden metal knowledge and provide extensive reference database to users. It provides users reliable information and knowledge to the greatest extent. If there is any copyright violation, please notify us through our contact details to delete such infringement content promptly.








 Most cadmium pigments are used in plastics. These pigments disperse well in most polymers to give good colouring and high opacity and tinting strength. The pigments are insoluble in organic solvents, have good resistance to alkalis and in most cases will remain colour fast for the life of the plastic. As a result, cadmium pigments have been used in a wide range of plastic products. Currently, their primary application is in complex polymers which are processed at higher temperatures and require the unique durability and technical performance of a cadmium pigment. Their use is almost mandatory in many nylons, acrylonitrile butadiene styrenes (ABS), polycarbonates, high density polyethylenes, silicone resins and other modern thermoplastic polymers processed at high temperatures which preclude the use of organic pigments and also most alternative inorganic pigments in the range of hues provided by cadmium. Cadmium pigmented engineering polymers such as ABS are widely used in products which include telephones, gas pipes and fittings, electricity cables, beverage crates and motor vehicle radiator fans. Pigments are usually incorporated in plastics in proportions of 0.01 to 0.75 per cent by weight.
Most cadmium pigments are used in plastics. These pigments disperse well in most polymers to give good colouring and high opacity and tinting strength. The pigments are insoluble in organic solvents, have good resistance to alkalis and in most cases will remain colour fast for the life of the plastic. As a result, cadmium pigments have been used in a wide range of plastic products. Currently, their primary application is in complex polymers which are processed at higher temperatures and require the unique durability and technical performance of a cadmium pigment. Their use is almost mandatory in many nylons, acrylonitrile butadiene styrenes (ABS), polycarbonates, high density polyethylenes, silicone resins and other modern thermoplastic polymers processed at high temperatures which preclude the use of organic pigments and also most alternative inorganic pigments in the range of hues provided by cadmium. Cadmium pigmented engineering polymers such as ABS are widely used in products which include telephones, gas pipes and fittings, electricity cables, beverage crates and motor vehicle radiator fans. Pigments are usually incorporated in plastics in proportions of 0.01 to 0.75 per cent by weight.
