- The toxicity of cadmium to human health and environment
 Cadmium is a non-essential and toxic element for humans. It is toxic at very low exposure levels and produce acute and chronic effects on health and the environment. Cadmium itself is likely to show a similar range of toxicological properties to cadmium chloride. However, there are some other cadmium compounds, including sulphide and cadmium pigments, for which the evidence in relation to cancer in humans is weaker. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen, and cadmium is recognized as a developmental toxicant and reproductive toxicant.
Cadmium is a non-essential and toxic element for humans. It is toxic at very low exposure levels and produce acute and chronic effects on health and the environment. Cadmium itself is likely to show a similar range of toxicological properties to cadmium chloride. However, there are some other cadmium compounds, including sulphide and cadmium pigments, for which the evidence in relation to cancer in humans is weaker. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen, and cadmium is recognized as a developmental toxicant and reproductive toxicant.- Cadmium isn’t capable of being decomposed in nature and will thus, once released into the environment, stay in circulation. Compared to other heavy metals, cadmium and cadmium compounds are relatively water soluble. Therefore, they are more easily absorbed and mobile, for instance, in soil, generally more bio-available and tend to bio-accumulate.
- Currently, due to the versatility of cadmium, industrial and consumer applications are all around us in our daily lives.
- Cadmium is released into the environment through mining and smelting operations (primarily zinc, lead, copper and cadmium), fuel combustion (in coal-, wood- and oil-fired boilers or in vehicle engines), incineration of municipal waste and sewage sludge, the spreading of sewage sludge, the disposal of metal-containing products and the application of phosphate fertilizer. Cadmium can also be released naturally through volcanic eruptions, forest fires and soil erosion.
- Cadmium particles travel in the form of dust or fall from the atmosphere in rain and settle, coming into our water supplies, soil and crops. For the general population, the most common routes of cadmium exposure are contaminated food and cigarette smoke.

- Crops for human consumption, particularly grains and cereal products— including rice, potatoes, root vegetables, leafy vegetables and tobacco— take up cadmium from the soil.
- Drinking water often holds a small amount of cadmium (average 1 part per billion); public notification is required when a water supply contains more than 5 parts per billion. Cadmium from improperly regulated hazardous waste sites, factories or incinerators can escape as dust or contaminate food and water supplies, exposing people who live nearby to elevated levels of cadmium.
- Chronic cadmium exposure produces a wide range of acute and chronic effects in humans. Attention is drawn to the following:
- Cadmium accumulates in the human body and especially in the kidneys. According to the current knowledge kidney damage (renal tubular damage) is probably the critical health effect. The main critical effects include an increased excretion of proteins in urine as a result of proximal tubular cell damage. The severity of the effect depends on the duration and magnitude of exposure.
- Skeletal damage is another critical effect of chronic cadmium exposure at levels somewhat higher than those for which kidney proteinuria is an early effects indicator .
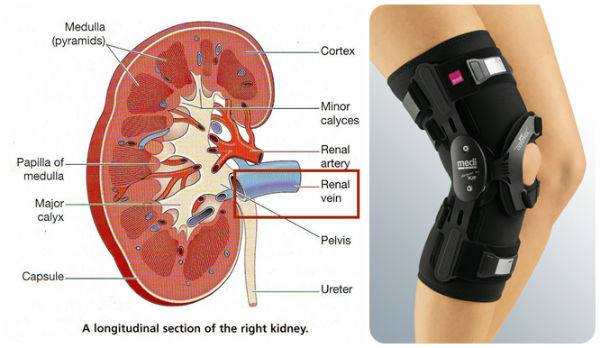
 High exposure can lead to lung cancer and prostate cancer. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen by the inhalation route. Epidemiological data from occupational settings confirm lungs being the primary target organ. Cadmium is not considered a carcinogen by ingestion.
High exposure can lead to lung cancer and prostate cancer. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen by the inhalation route. Epidemiological data from occupational settings confirm lungs being the primary target organ. Cadmium is not considered a carcinogen by ingestion.- Drinking water often holds a small amount of cadmium (average 1 part per billion); public notification is required when a water supply contains more than 5 parts per billion. Cadmium from improperly regulated hazardous waste sites, factories or incinerators can escape as dust or contaminate food and water supplies, exposing people who live nearby to elevated levels of cadmium.
- Low-dose exposure to elevated levels of cadmium over a long period of time has different health consequences than a single high dose exposure. Acute health effects, such as flu-like symptoms, intestinal tract ailments and lung irritation, can be caused by intense short-term exposure.
 • Atmospheric deposition seems to be causing the content of cadmium in agricultural top soil to continually increase, which in time will be reflected in an increased human intake via foodstuffs and therefore in an increased human risk of kidney damages, and other effects related to cadmium.
• Atmospheric deposition seems to be causing the content of cadmium in agricultural top soil to continually increase, which in time will be reflected in an increased human intake via foodstuffs and therefore in an increased human risk of kidney damages, and other effects related to cadmium. - • In the marine environment levels of cadmium may significantly exceed background levels, causing the potential for serious effects on marine animals and in particular birds and mammals.
- • Significant quantities of cadmium are continually being stockpiled in land-fills and other deposits and represent a significant potential for future releases to the environment.
- The dominant sources of atmospheric emissions will vary depending on the region or country considered. Non-ferrous metal production as well as combustion of coal and oil and waste incineration should be considered important sources. The principal sources of cadmium input to the marine environment include atmospheric deposition, domestic waste water and industrial discharges. Emissions to air as well as water on an international scale seem to be lowering, due to improved flue gas and waste water treatment.
- The environmental consequences and the toxicity of cadmium call for a global initiative aimed at minimizing human and environmental effects of the ongoing cadmium emissions. The relevance of considering a global initiative comes, furthermore, from the fact that cadmium used intentionally in products is traded globally and that effective risk reduction measures thus must be seen in a global context.
- Global efforts addressing cadmium may include a phasing-out of cadmium in products, as well as global agreements on improved emissions control relating to both air and water emissions. Adequate substitutes exist for many of the applications for which cadmium is still being used.
- The current low world market price of cadmium motivates the development of new applications that over time may develop into new sources of environmental emissions not covered by existing regulations.
-
About us
Contact us
Make a suggestion
- Metalpedia is a non-profit website, aiming to broaden metal knowledge and provide extensive reference database to users. It provides users reliable information and knowledge to the greatest extent. If there is any copyright violation, please notify us through our contact details to delete such infringement content promptly.
 Cadmium is a non-essential and toxic element for humans. It is toxic at very low exposure levels and produce acute and chronic effects on health and the environment. Cadmium itself is likely to show a similar range of toxicological properties to cadmium chloride. However, there are some other cadmium compounds, including sulphide and cadmium pigments, for which the evidence in relation to cancer in humans is weaker. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen, and cadmium is recognized as a developmental toxicant and reproductive toxicant.
Cadmium is a non-essential and toxic element for humans. It is toxic at very low exposure levels and produce acute and chronic effects on health and the environment. Cadmium itself is likely to show a similar range of toxicological properties to cadmium chloride. However, there are some other cadmium compounds, including sulphide and cadmium pigments, for which the evidence in relation to cancer in humans is weaker. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen, and cadmium is recognized as a developmental toxicant and reproductive toxicant.

 High exposure can lead to lung cancer and prostate cancer. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen by the inhalation route. Epidemiological data from occupational settings confirm lungs being the primary target organ. Cadmium is not considered a carcinogen by ingestion.
High exposure can lead to lung cancer and prostate cancer. The US Environmental Protection Agency (EPA) now classifies cadmium as a known human carcinogen by the inhalation route. Epidemiological data from occupational settings confirm lungs being the primary target organ. Cadmium is not considered a carcinogen by ingestion. • Atmospheric deposition seems to be causing the content of cadmium in agricultural top soil to continually increase, which in time will be reflected in an increased human intake via foodstuffs and therefore in an increased human risk of kidney damages, and other effects related to cadmium.
• Atmospheric deposition seems to be causing the content of cadmium in agricultural top soil to continually increase, which in time will be reflected in an increased human intake via foodstuffs and therefore in an increased human risk of kidney damages, and other effects related to cadmium. 