 Cadmium is mainly a byproduct of beneficiating and refining of zinc metal from sulfide ore concentrates. The mined zinc ores are crushed and ground to liberate the zinc sulfide particles from the waste host rock. The ground ore is usually treated by a differential flotation process to separate the zinc-bearing particles from the waste rock, yielding a high-grade zinc concentrate and a waste product calling tailings. The cadmium content of the zinc concentrate is usually around 0.3% to 0.5%.
Cadmium is mainly a byproduct of beneficiating and refining of zinc metal from sulfide ore concentrates. The mined zinc ores are crushed and ground to liberate the zinc sulfide particles from the waste host rock. The ground ore is usually treated by a differential flotation process to separate the zinc-bearing particles from the waste rock, yielding a high-grade zinc concentrate and a waste product calling tailings. The cadmium content of the zinc concentrate is usually around 0.3% to 0.5%.- An estimated 90% to 98% of the cadmium present in zinc ores is recovered in the mining and beneficiating stages of the extraction process. Figure 1 shows a schematic flow of mining and beneficiating a typical lead-zinc ore.
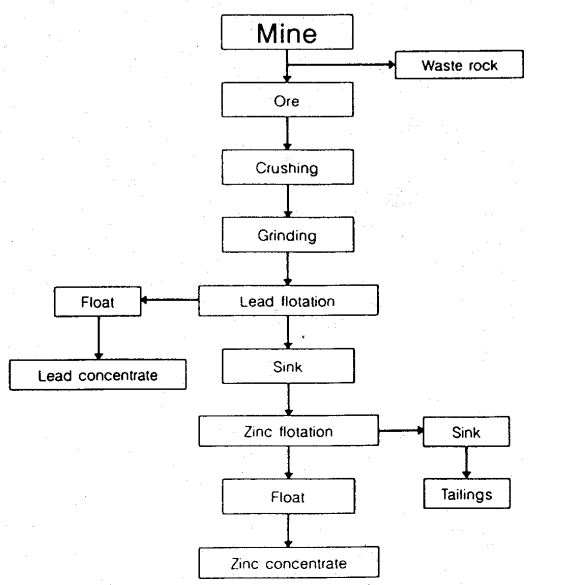
- Figure 1. Schematic flowchart of mining and beneficiating a typical lead-zinc ore
- Refining of zinc and its cadmium content, can be accomplished by treating the zinc concentrates and/or zinc-bearing secondary materials using either a hydrometallurgical or pyrometallurgical process. In both processes, the concentrate is converted from zinc sulfide to zinc oxide by roasting, and at the same time most of the sulfur is removed as sulfur dioxide (SO2). The SO2 offgas is stripped of all entrapped dust and other impurities and then converted to sulfuric acid in an acid plant.
- Hydrometallurgical process
- In the hydrometallurgical process, zinc, copper and cadmium are dissolved in the sulfuric acid leach of the roasted zinc ore. The copper and cadmium are among the most common interfering impurities that are removed before the purified solution is subjected to electrolysis for zinc recovery. Copper is precipitated from the solution using a determined amount of zinc dust. Most of the cadmium is precipitated in a second zinc dust addition, and any remaining dissolved cadmium is precipitated by a third stage of zinc dust addition. The purified zinc sulfate solution is sent to the cellroom and metallic zinc is recovered from the solution by electrowinning. The cadmium precipitate is sent to the cadmium plant where it is filtered and formed into a cake containing cadmium, zinc, and minor amounts of copper and lead. Through various steps of purification the impurities are separated and a sufficiently pure cadmium sponge is dissolved in sulfuric acid. Metallic cadmium is recovered by electrolyzing this solution where cadmium is deposited on cathodes. After deposition, the cathodes are removed from the cells and stripped, the cadmium metal is melted and cast into desired shapes. Figure 2 is a schematic flowchart of a hydrometallurgical refining process to produce zinc as well as cadmium metal from zinc concentrates.
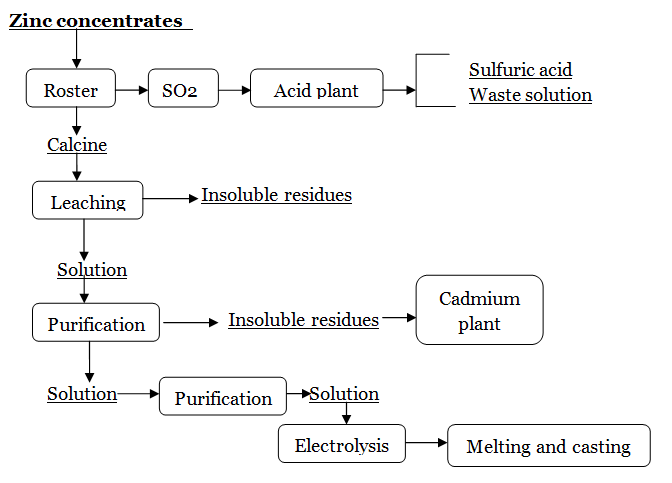
- Figure 2. Schematic flowchart for hydrometallurgical refining of zinc concentrates
- Pyrometallurgical Process
- In the pyrometallurgical process, cadmium is volatilized during the roasting and sintering of zinc concentrates, and the resultant fume and dust are collected as flue dust in baghouses or electrostatic precipitators. The initial cadmium content of the flue dust can be as high as 10%. A great deal of cadmium collects with the zinc metal and may be removed by refining of zinc by fractional distillation (the boiling point of cadmium is 767℃ and that of zinc and cadmium metal from zinc concentrates.
- The following process is usually used to obtain cadmium produced from flue dust collected at lead or copper smelters. Concentrates of copper, and especially lead, contain considerable amounts of cadmium. In copper smelters, the cadmium reports in flue dusts, which are collected and recycled through the smelter system to upgrade the cadmium content. At the lead smelters, the cadmium is fumed off and collected in the blast furnace baghouses. The baghouse dust is recycled to upgrade the cadmium content and later used as feed material for the cadmium refinery plant.
- The cadmium-upgraded dusts are charged into a tank and dissolved with sulfuric acid. The resulting solution is filtered to remove impurities and obtain a purified cadmium sulfate solution. Next, metallic cadmium, called sponge because of its appearance, is precipitated from the solution using zinc dust. The sponge is usually briquetted, remelted, and cast into ingots. Some plants produce cadmium oxide and/or metallic cadmium powder. Cadmium oxide is produced by melting the ingots and keeping a controlled oxidizing atmosphere in the retort. To produce metal powder, the melted ingots in the retort are kept under an inert atmosphere while cadmium is distilled into a condenser as metallic powder.
- The USBM estimated that about 95% of the cadmium content of zinc, lead, and copper concentrates is recovered in the smelting and refining process stages.
- Commercial grades of cadmium have 99.95%to 99.96% minimum purity, but for special applications such as semi-conductors, grades up to 99.9999% purity are produced by vacuum distillation. Cadmium metal is produced in a variety of shapes such as slabs, ingots and sticks, which are used in alloying, pigments, and in production of cadmium oxide. Balls and sheets are used for plating anodes.
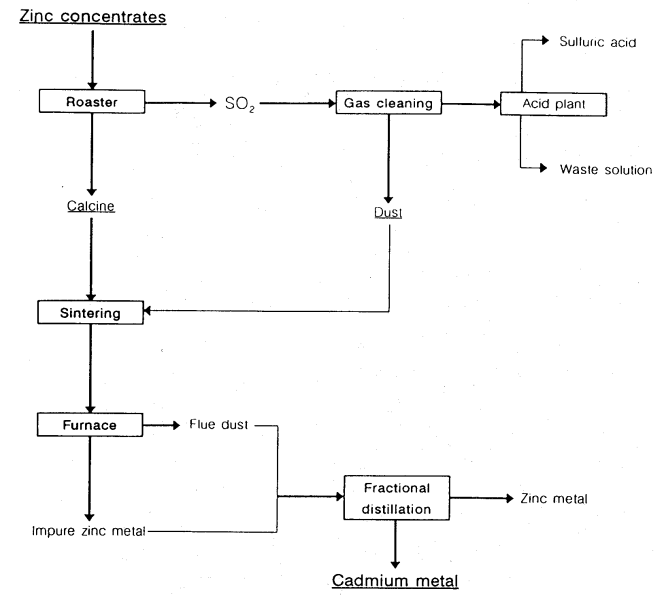
- Figure 3. Schematic flowchart for pyrometallurigical refining of zinc concentrates
-
About us
Contact us
Make a suggestion
- Metalpedia is a non-profit website, aiming to broaden metal knowledge and provide extensive reference database to users. It provides users reliable information and knowledge to the greatest extent. If there is any copyright violation, please notify us through our contact details to delete such infringement content promptly.
 Cadmium is mainly a byproduct of beneficiating and refining of zinc metal from sulfide ore concentrates. The mined zinc ores are crushed and ground to liberate the zinc sulfide particles from the waste host rock. The ground ore is usually treated by a differential flotation process to separate the zinc-bearing particles from the waste rock, yielding a high-grade zinc concentrate and a waste product calling tailings. The cadmium content of the zinc concentrate is usually around 0.3% to 0.5%.
Cadmium is mainly a byproduct of beneficiating and refining of zinc metal from sulfide ore concentrates. The mined zinc ores are crushed and ground to liberate the zinc sulfide particles from the waste host rock. The ground ore is usually treated by a differential flotation process to separate the zinc-bearing particles from the waste rock, yielding a high-grade zinc concentrate and a waste product calling tailings. The cadmium content of the zinc concentrate is usually around 0.3% to 0.5%.


