- Lead smelting and lead alloy classification
- Lead metal can be classified as either primary or secondary. Primary lead is produced directly from mined lead ore whereas secondary lead is produced from scrap lead products (such as automobile batteries) which have been recycled. Total annual production is approximately 8 million tonnes, half of which is primary lead. It is rare to find pure deposits of lead in nature though. The majority of the deposits are mixtures of minerals, hence lead ore is usually obtained as a byproduct of other metal mining such as zinc, silver or copper. In fact, lead ore is a main source of silver and contributes substantially towards the world's total output. The most common lead ore is galena (PbS), which contains 86.6% lead. Other common varieties include cerussite (PbCO3) and angelsite (PbSO4).
- Source: http://www.worldresourcesforum.org/resource-snapshot-6-lead
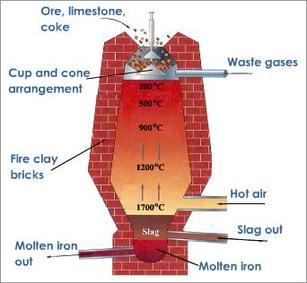 A major and primary Lead mineral is galena (PbS) which comprises of 86.6% of lead. In order to smelt this mineral, a blast furnace is needed to be used. A blast furnace is an enormous oven which is used to accomplish the smelting processes. The process in which galena is smelted requires two important chemical reactions to occur within the furnace.
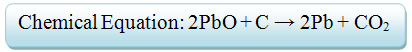
A major and primary Lead mineral is galena (PbS) which comprises of 86.6% of lead. In order to smelt this mineral, a blast furnace is needed to be used. A blast furnace is an enormous oven which is used to accomplish the smelting processes. The process in which galena is smelted requires two important chemical reactions to occur within the furnace.- 1) The galena is roasted (reacts with O2) in order to remove the sulfur component of the metal sulfide. Roasting is a method where a sulfide ore (i.e an ore containing PbS) is heated in air which converts the metal sulfide to either a metal oxide or a metal itself.
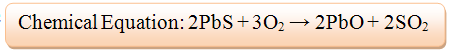
- 2) The newly formed Lead (II) Oxide subsequently reacts with coke to attain refined Lead. Coke is a pure form of coal that contains carbon and is essential in the extraction of metals from their oxides.
- There is another process in which the galena mineral can be smelted through. It also requires two chemical reactions to occur within the furnace.
- 1) Due to the sulfur content, carbon from the coke will not be able to reduce lead. Therefore, the mineral must be roasted to oxidize sulfur and create a metal oxide out of galena.
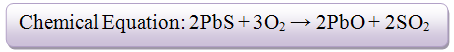
- 2) Raw ore is then added to the products and is heated further.
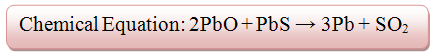
- Source: http://siddikabasemetalsmelting.weebly.com/lead-smelting.html Chemical Reactions of Lead Smelting-Base Metal Smelting
- This simply shows the general theory of the pyrometallurgy of lead. The details are as follows:
- First of all, the ore must be concentrated to separate the lead ore from the zinc ore, for example. It turns out to be possible to do this with flotation. A substance is added that wets the zinc ore, allowing it to sink to the bottom, but does not wet the galena, allowing it to be caught up in a foam that floats on the surface of the water. The result is not just lead sulphide, but also the sulphides of copper, iron, zinc, antimony and arsenic. The enriched ore is then roasted in ovens to drive off as much of the sulphur as possible. The roasted ore must be ground and sintered to put it in the form of porous chunks that allow gases to pass through freely, and will not collapse into a thick, impervious layer in the blast furnace. Lead ores are such that these two operations are best combined into one simultaneous roasting-sintering process that produces a sinter ready for the lead blast furnace.
- The second stage of smelting can take place in an ore hearth, or a larger blast furnace. The sintered ore is charged, with coke, limestone flux, and other additives depending on the impurities present. The products that accumulate at the bottom are lead, matte (containing iron and copper), speiss (containing iron and arsenic), and slag (containing the silicates, zinc, iron and calcium). Cold air is blown in at the bottom, and flue dust and gases come out the top. The lead bullion from Mississippi valley ores is called "soft" lead and is pure enough for most uses without further treatment. The other by-products are treated to separate their valuable constituents. Zinc, incidentally, does not dissolve in molten lead, and can be added to extract impurities by differential solubility.
 Lead is sold as soft lead, 99.90% pure, common lead (lead that has been desilvered), 99.85% pure, and corroding lead (for paint), 99.94% pure. Hard lead is alloyed with 6%-18% antimony, which increases the strength of the lead. The addition of only 1% Sb or 3% Sn increases the strength by 50%. Hard lead is used for battery plates. Terne plate is heavy sheet steel coated with a lead-tin alloy. 75-25 and 50-50 Pb-Sn alloys are used.
Lead is sold as soft lead, 99.90% pure, common lead (lead that has been desilvered), 99.85% pure, and corroding lead (for paint), 99.94% pure. Hard lead is alloyed with 6%-18% antimony, which increases the strength of the lead. The addition of only 1% Sb or 3% Sn increases the strength by 50%. Hard lead is used for battery plates. Terne plate is heavy sheet steel coated with a lead-tin alloy. 75-25 and 50-50 Pb-Sn alloys are used. - Source: http://mysite.du.edu/~jcalvert/phys/lead.htm#Prod-Lead
- According to their properties and applications, lead alloys can be classified into: corrosion resistant alloys, battery alloys, solder alloys, printing alloys, bearing metal and mould alloys etc.
- Classification of lead ingots (China)
- Lead ingots can generally be divided into small ingots and big ingots.
- Small ingots are rectangular trapezoidal, the bottom features baling grooves and both ends have a prominent ear. Single weight:48kg±3kg、42kg±2kg、40kg±2kg、24kg±1kg.
- Big ingots are trapezoidal and have a tee at the bottom of the convex block with grab crane slots on both sides. Single weight:950 kg±50kg、500 kg±25kg.
-
About us
Contact us
Make a suggestion
- Metalpedia is a non-profit website, aiming to broaden metal knowledge and provide extensive reference database to users. It provides users reliable information and knowledge to the greatest extent. If there is any copyright violation, please notify us through our contact details to delete such infringement content promptly.
 A major and primary Lead mineral is galena (PbS) which comprises of 86.6% of lead. In order to smelt this mineral, a blast furnace is needed to be used. A blast furnace is an enormous oven which is used to accomplish the smelting processes. The process in which galena is smelted requires two important chemical reactions to occur within the furnace.
A major and primary Lead mineral is galena (PbS) which comprises of 86.6% of lead. In order to smelt this mineral, a blast furnace is needed to be used. A blast furnace is an enormous oven which is used to accomplish the smelting processes. The process in which galena is smelted requires two important chemical reactions to occur within the furnace.

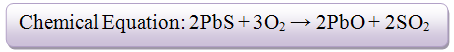

 Lead is sold as soft lead, 99.90% pure, common lead (lead that has been desilvered), 99.85% pure, and corroding lead (for paint), 99.94% pure. Hard lead is alloyed with 6%-18% antimony, which increases the strength of the lead. The addition of only 1% Sb or 3% Sn increases the strength by 50%. Hard lead is used for battery plates. Terne plate is heavy sheet steel coated with a lead-tin alloy. 75-25 and 50-50 Pb-Sn alloys are used.
Lead is sold as soft lead, 99.90% pure, common lead (lead that has been desilvered), 99.85% pure, and corroding lead (for paint), 99.94% pure. Hard lead is alloyed with 6%-18% antimony, which increases the strength of the lead. The addition of only 1% Sb or 3% Sn increases the strength by 50%. Hard lead is used for battery plates. Terne plate is heavy sheet steel coated with a lead-tin alloy. 75-25 and 50-50 Pb-Sn alloys are used. 